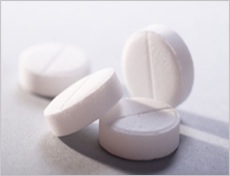
MAPS’ top priority project is funding clinical trials of methylenedioxymethamphetamine (MDMA) as a therapeutic tool to assist psychotherapy for the treatment of Posttraumatic Stress Disorder (PTSD) and other illnesses. Preliminary studies have shown that MDMA in conjunction with psychotherapy can help people overcome PTSD. MDMA has empathogenic effects, and it is also known as the popular drug Ecstasy (although “Ecstasy” does not always contain pure MDMA). In laboratory studies, MDMA has been proven sufficiently safe for human consumption when taken a limited number of times in moderate doses.
MAPS is in the midst of a 10 year – $10 million plan to make MDMA into a government-approved prescription medicine. For-profit pharmaceutical companies are not interested in developing MDMA into a medicine, because the patent for MDMA has expired. Companies cannot profit off of MDMA because it is only administered a limited number of times, unlike most medications for mental illnesses that are taken on a daily basis. Consequently MAPS is the only organization funding clinical trials of MDMA-assisted psychotherapy in the world.
MAPS is studying whether MDMA-assisted psychotherapy has the potential to heal traumas caused by sexual assault, war, violent crime, and other causes.
Below is comprehensive and updated information about MAPS efforts to develop MDMA into a government-approved prescription medicine, as well as useful information for understanding the benefits and risks of MDMA use.
[From MDMA Research]